Suriname boost will not suffice, Indian Pharmacopoeia needs more global acceptances
Global discrimination is visible against Indian drugs because of affordable prices
image for illustrative purpose
In a clear indication of the expanding reach of the Indian Pharmacopoeia (IP) as a standard book in other importing countries, the Republic of Suriname, a small country on the northeastern coast of South America, has officially recognized IP in the category. A MoU was signed recently between the Indian Pharmacopoeia Commission (IPC) and the Health Ministry of Suriname for recognition of IP as a book of standard. IPC had earlier submitted proposals to various countries to increase its efforts towards recognition and acceptance of IP for a similar recognition.
In effect Suriname became the fifth country to recognize IP after Afghanistan, Ghana, Nepal and Mauritius in that order.
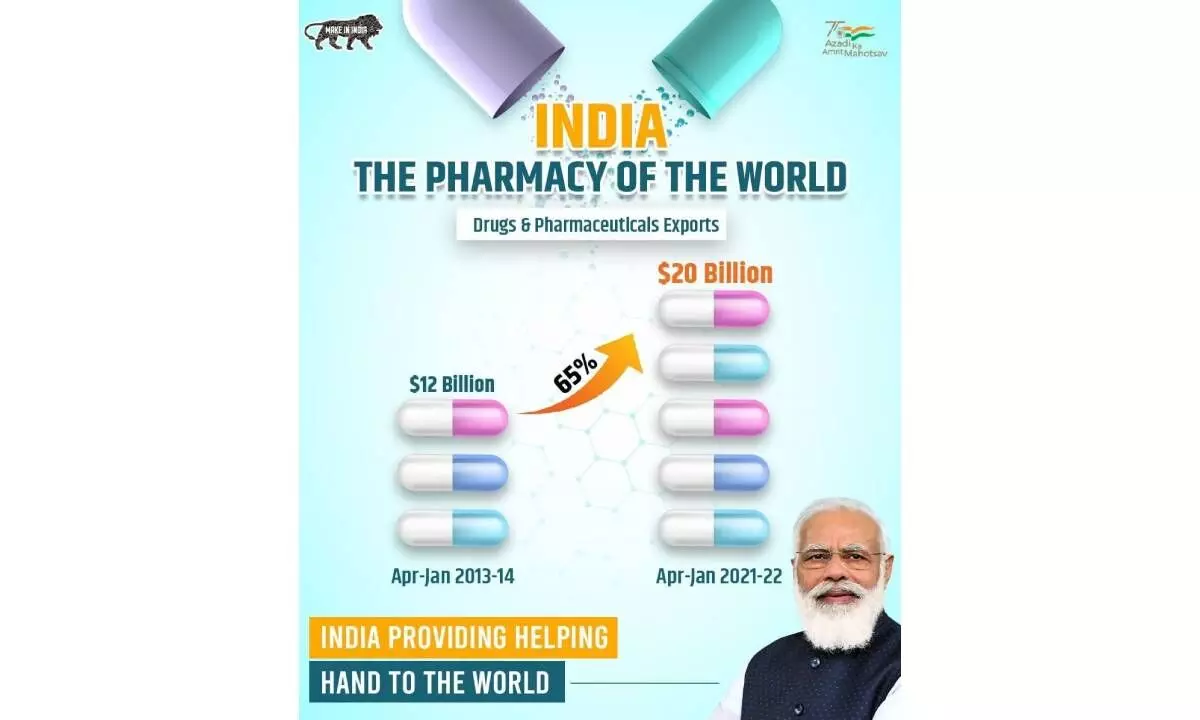
It is quite ironic that even though India exports, mainly generics, to more than 250 countries and one out of the three medicines consumed by a patient in any part of the globe is from India, IP is still not recognized by many.
On the other hand, US Pharmacopoeia (USP), British Pharmacopoeia (BP), and European Pharmacopoeia (EP) are accepted by all of them. Medicines to the US, the UK and EU countries are supplied by India, but they are reluctant to recognize IP. Its acceptance by more countries will be a huge relief for Indian pharma and merchant exporters. For the merchant exporters, medicinal products in IP can be exported as they are without any further tests and it does away with applying for a separate production method. Like in the domestic market, medicines manufactured in IP can be bought and exported if more countries treat them like their locally manufactured drugs.
It is a fact that IP standards ensure quality and safety of drugs by providing analytical methods and appropriate limits for testing and assessing the raw materials, excipients and finished formulations. But, even in Gulf countries like UAE and other GCC countries, more than 40 per cent of the medicines used by the people are made by Indian companies, but, all these medicines are not manufactured in IP standards but in the pharmacopoeia standards of the US or UK or the European Union.
For IP to get accepted as an international pharmacopoeia standard, the government of India has to convince other countries about the quality attributes of the Indian medicines. Discrimination can be seen in the world market against Indian made drugs because of their affordable prices, though they do not compromise on quality. The quality of Indian medicines is accepted by all, but the IP standards are not accepted as equivalent to the standards of US, British or pharmacopoeia of European nations.
No doubt, the Union Health Ministry has been periodically taking several steps to get the deserving recognition for IP globally. Besides, the ministry has been upgrading IP to improve the quality of drugs produced in the country. In this regard, the ministry released the ninth edition of IP last July, containing 92 new monographs for drugs, 12 new general chapters, 1,245 monographs for formulations, 930 monographs for active pharmaceutical ingredients (APIs) as well as dissolution specifications for all prolonged release formulations. Undoubtedly, it was a step forward to delivering quality medicines.
Several monographs and general chapters have also been revised to update them as per current global requirements and to harmonize with other pharmacopoeias like USP, BP and EP. The harmonization of standards with global standards is expected to help IP getting recognized and accepted in foreign countries.
The central government is now in talks with UAE to include IP in the standards of pharmaceuticals in a bid to boost exports to the Emirates. The Centre should leave no stone unturned to expand the reach of IP. The government should sensitize the international regulators about the quality of medicines manufactured on IP standards. More and more acceptance of IP will come as a shot in the arm, albeit belated, for the Indian pharmaceutical exporters.
(The author is freelance journalist with varied experience in different fields)

